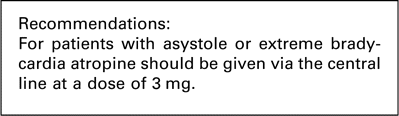-
PDF
- Split View
-
Views
-
Cite
Cite
Joel Dunning, Alessandro Fabbri, Philippe H. Kolh, Adrian Levine, Ulf Lockowandt, Jonathan Mackay, Alain J. Pavie, Tim Strang, Michael I.M. Versteegh, Samer A.M. Nashef, on behalf of the EACTS Clinical Guidelines Committee, Guideline for resuscitation in cardiac arrest after cardiac surgery, European Journal of Cardio-Thoracic Surgery, Volume 36, Issue 1, July 2009, Pages 3–28, https://doi.org/10.1016/j.ejcts.2009.01.033
Close - Share Icon Share
Summary
The Clinical Guidelines Committee of the European Association for Cardio-Thoracic Surgery provides this professional view on resuscitation in cardiac arrest after cardiac surgery. This document was created using a multimodal methodology for evidence generation including the extrapolation of existing guidelines from the International Liaison Committee on Resuscitation where possible, our own structured literature reviews on issues particular to cardiac surgery, an international survey on resuscitation hosted by CTSNet and manikin simulations of potential protocols. This protocol differs from existing generic guidelines in a number of areas, the most import of which are the following: successful treatment of cardiac arrest after cardiac surgery is a multi-practitioner activity with six key roles that should be allocated and rehearsed on a regular basis; in ventricular fibrillation, three sequential attempts at defibrillation (where immediately available) should precede external cardiac massage; in asystole or extreme bradycardia, pacing (where immediately available) should precede external cardiac massage; where the above measures fail, and in pulseless electrical activity, early resternotomy is advocated; adrenaline should not be routinely given; protocols for excluding reversible airway and breathing complications and for safe emergency resternotomy are given. This guideline is subject to continuous informal review, and when new evidence becomes available.
1 Introduction
The European Resuscitation Council (ERC) issued the latest edition of guidelines for resuscitation in December 2005 [1–9]. For the first time this included a detailed section on the resuscitation of patients with cardiac arrest after cardiac surgery [7]. This document has stimulated many clinicians managing cardiac surgical patients to evaluate more carefully how cardiac arrests are managed in their own units. There is recognition that, after cardiac surgery, certain variables may dictate differences in the management of cardiac arrest when compared to other situations.
Every year, over 250,000 patients have cardiac surgery in some 450 centres in Europe [10]. The incidence of cardiac arrest after cardiac surgery is around 0.7–2.9% [11–19] and has reduced in recent years. The most remarkable statistic regarding these patients is the relatively good outcome with 17–79% of patients suffering a cardiac arrest surviving to hospital discharge, a far higher proportion than can be hoped for when cardiac arrest occurs in other settings. The reason for this superior survival is the high incidence of reversible causes for the arrest. Ventricular fibrillation (VF) accounts for the rhythm in 25–50% of cases and, in the intensive care unit (ICU) setting; this is immediately identified and treated. In addition, tamponade and major bleeding account for many arrests and both conditions may be quickly relieved by prompt resuscitation and emergency resternotomy where appropriate.
Because many patients may potentially be saved by prompt treatment, ICU staff must be well versed in managing cardiac arrests. Practising protocol-based arrest management has been shown to halve the time to chest reopening and reduce complications in the conduct of the resternotomy after cardiac surgery [20–22].
The protocol presented here addresses many issues particular to our specialty including the timing of emergency resternotomy, the number of attempts at defibrillation before reopening, the administration of adrenaline, ventilator, infusion and pacemaker settings, emergency resternotomy sets, the use of the intra-aortic balloon pump (IABP), and cardiac arrests on the ward and in special circumstances.
This protocol applies to all patients in the ICU and includes paediatric patients and transplant patients, but not patients undergoing thoracic surgery unless by a sternotomy. Issues regarding the treatment of patients in mixed specialty wards are discussed.
It should be noted that the International Liaison Committee on Resuscitation (ILCOR) is due to update its guidelines in 2010, and the European Resuscitation Council will update its 2005 guidelines on the basis of ILCOR’s recommendations. We hope that this document will assist in informing and developing these updates.
2 Methods
In 2007, the Audit and Guidelines Committee (now the Clinical Guideline Committee) of the European Association for Cardio-Thoracic Surgery (EACTS) launched a project to create a set of clear clinical guidelines to apply specifically to resuscitation after cardiac surgery. We used a variety of methodologies in creating these guidelines in order to obtain as wide a range of opinions as possible from the cardiothoracic surgical community and beyond.
Where there was a published body of evidence to guide practice, a structured literature review was performed and published in the ICVTS. These topics were open for website commentary for 2 months, and comments were published together with the literature review in the full print edition of this journal.
A survey was conducted on CTSnet (www.ctsnet.org) from January to June 2008 on a wide range of issues pertaining to resuscitation of cardiac surgical patients and this was used to guide decision-making by the committee.
Finally, a range of cardiac arrest protocols were tested on manikins with personnel from cardiothoracic surgery and ICU teams and these tests were recorded on video, analysed and evaluated for usability and practicality during 17 courses teaching resuscitation in patients after cardiac surgery [20].
These guidelines were created using the process of evidence generation described for the International Liaison Committee on Resuscitation combined with our own methodology used previously and published in the ICVTS [23,24]. The review process features a full literature search and description of the search strategy together with the findings, a summary of the papers found and a conclusion. Worksheets from ILCOR are used where no relevant new studies could be found for cardiac surgery, but for all topics directly relevant to this guideline, a topic review was published in full in the ICVTS together with the submitted comments. This protocol has been successfully used to create guidelines for EACTS previously [25,26].
2.1 Levels of evidence (LOE) and grading recommendations
This guideline uses the ILCOR protocol for assessment of levels of evidence [27]. Individual studies are graded as follows:
LOE 1: Randomised controlled trials (RCT) (or meta-analyses of RCTs).
LOE 2: Studies using concurrent controls without true randomisation.
LOE 3: Studies using retrospective controls.
LOE 4: Studies without a control group (case series).
LOE 5: Studies not directly related to the specific patient population.
The quality of each study is also assessed in the following manner:
Good studies have most or all of the relevant quality items.
Fair studies have some of the relevant quality items.
Poor or unsatisfactory studies have few of the relevant quality items.
3 Proposed protocol for cardiac surgical patients in ICU
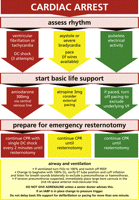
The proposed modification of the European Resuscitation Council advanced life support cardiac arrest algorithm to be applied in cardiac arrest after cardiac surgery is presented in Fig. 1 . We recommend that this protocol should be used in the ICU. It is not recommended for use outside of this setting. This protocol should be used in preference to the protocol currently recommended for patients with cardiac arrest which does not follow cardiac surgery [4]. Major differences between the protocols are addressed below.
EACTS guideline for resuscitation of a patient who arrests after cardiac surgery.
3.1 Cardioversion
One major change is the speed and vigour with which cardioversion is attempted. Before this guideline, a patient in ventricular fibrillation after cardiac surgery was to receive a single attempt at cardioversion followed immediately by cardiopulmonary resuscitation (CPR) including external cardiac massage (ECM) for 2 min. Thereafter, the rhythm is reassessed and CPR ceased if evidence of a cardiac output is found. Cardiac surgical patients are sufficiently different from other patients for us to recommend an important departure from this guideline. Firstly the cardiac surgical patient in ICU usually has continuous arterial line, central venous line, pulse oximetry and ECG monitoring. Thus the arrest will be immediately identified, making early cardioversion more likely to be successful in restoring cardiac output without any ECM. Secondly immediate ECM is unnecessary in certain cases, and its use after cardiac surgery should be minimised due to the risk of trauma to the surgical site [28]. Thirdly, if cardioversion is unsuccessful, rapid resternotomy may be indicated. Thus three successive attempts at defibrillation will increase the likelihood of restoring cardiac output after a VF arrest while minimising the delay to chest reopening where indicated as well as reducing the risk of trauma to recently operated cardiac structures and suture lines. As recognition of VF is immediate, a prolonged period without cardiac output or CPR is unlikely, and the rationale for the traditional recommendation is less likely to be pertinent here [3].
Of note, we recommend biphasic rather than uniphasic defibrillation at the recommended optimal strength for your particular defibrillator, in line with ERC recommendations. This optimal strength may vary between 150 J and 360 J according to the manufacturer of your defibrillator and your local resuscitation officer should be contacted if you have any doubts as to the optimal strength of defibrillation in your hospital.
3.2 Identification of arrest and initiation of basic life support in the ICU
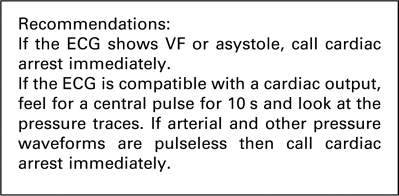
Patients in the ICU are highly monitored and often intubated and ventilated. A potential cardiac arrest is most likely therefore to be signalled by monitoring alarms. If you are the first person alerted to the possibility of an arrest you should immediately put your hand onto a central pulse (such as the femoral or carotid pulse) for up to 10 s. During this time, you should also check the arterial line for position and look at the other traces on the monitor. In a cardiac arrest, not only will the arterial line show no pulsatility, but also the central venous pressure (CVP) line, the pulse oximetry trace and the pulmonary artery (PA) pressure trace will be flat. An ECG trace looking like sinus rhythm in the absence of pulsatile traces from the other monitoring should be considered a cardiac arrest.
In contrast, if there is a palpable central pulse, and if CVP, oximetry and PA pressure waveforms are present then a cardiac arrest may be excluded and the integrity of arterial line should be assessed. A non-invasive blood pressure reading should be taken.
If after 10 s there is no palpable central pulse and the arterial, CVP, PA and oximetry waveforms are flat, you should immediately instigate the cardiac arrest protocol. You should also loudly and clearly shout for help, for example:
‘cardiac arrest bed 4!’
3.3 Doubtful diagnosis of cardiac arrest
If in doubt feel for a central pulse for 10 s and look at all traces. If there has been an arrhythmic arrest and the ECG is incompatible with an output (VF or asystole) then the diagnosis is straightforward and the arrest protocol can be initiated immediately without feeling for a pulse. An ECG lead displacement will cause a flat-line electrical trace with pulsatile pressure traces, and therefore will not simulate VF or asystole.
Sometimes there is a viable ECG and the arterial waveform has been gradually diminishing as the blood pressure falls. Assuming that the arterial line is functioning well (CVP, PA and oximetry trace amplitudes also diminishing) then immediate expert assistance should be sought, but cardiac arrest should not be called and the protocol not instituted until the arterial impulse is absent and all pressure waves become flat.
3.4 Basic life support: external cardiac massage
If you have witnessed the change of rhythm to VF or pulseless VT then ECM may be delayed until three shocks have been given if a defibrillator is rapidly available (within 1 min). Otherwise, ECM should be immediately initiated by interlocking your fingers and applying pressure in the middle of the sternum with your arms straight to depress the sternum 4–5 cm in depth. You should perform chest compressions at a rate of 100 beats/min.
In the ICU setting you will be able to assess how effective your compressions are by looking at the arterial trace on the monitor. You should aim for the ‘systolic’ impulse over 60 mmHg. If increasing the rate of compression improves the mean blood pressure on the monitoring screen then you should increase the rate of compression [29,30]
Inability to achieve an acceptable compression-generated blood pressure may indicate that the cause of the arrest warrants immediate emergency resternotomy (massive bleeding or tamponade or tension pneumothorax) and chest reopening should be expedited.
3.5 Basic life support: airway
In the ICU setting, assistance should be immediate and thus the second person to attend should address the airway and breathing. If the patient is not intubated then the second person to attend the arrest should administer 100% oxygen with a bag/valve/mask with 2 breaths every 30 compressions.
However, most patients will be intubated and ventilated. If you are the second person to attend the arrest then your most important role is immediately to increase the oxygen on the ventilator to 100%. After this, removing positive end-expiratory pressure (PEEP) is also recommended.
Confirmation of airway patency and bilateral air entry is important, although an acute airway or ventilator problem is an uncommon cause for cardiac arrest in the ICU. The possibility of a tension pneumothorax must also be considered as a cause for the arrest. The following steps are useful in ensuring a satisfactory airway and ventilation:
Check the position of the endotracheal tube.
Listen for any air excursion around the tube, and that the cuff is inflated.
Look at the chest for bilateral expansion.
Listen with a stethoscope for bilateral air entry.
Capnography may give valuable information about ET tube position/patency and ventilation.
We recommend that the ventilator is disconnected and breaths are administered with a bag/valve connected to 100% oxygen. Bag/valve ventilation will allow a rough assessment of lung compliance. If you are not happy doing this, it is safer to wait with the oxygen set to 100% for an anaesthetist to arrive. If you do use the bag/valve to ventilate the patient and you are happy with the air entry to both lungs on inflation and also on auscultation then the patient may safely be returned onto the ventilator.
If your examination indicates that a tension pneumothorax is a possibility, you should immediately place a large bore cannula into the 2nd intercostal space, anterior mid-clavicular line. If you are correct, the arrest may resolve. If you are not correct but the pleura are open on this side with a drain, you will not create a pneumothorax. If the pleura are closed on that side or if in doubt, a drain is indicated or the pleura may be directly opened at resternotomy.
If you are unable to inflate the lungs with the bag/valve, and a sucker will not pass down the ET tube, then ET tube occlusion or malpositioning should be suspected. The ET tube should be immediately removed and a bag/valve/mask with airway adjuncts used to maintain the airway.
3.6 Infusions and syringe drivers
We found no cases of arrest after cardiac surgery being caused by incorrect administration of drugs by a syringe driver or infusion pump although theoretically cardiac arrest may result from this. In addition, inadvertent flushing of a vasodilator or residual drug in the lumen of a central line is a conceivable cause of arrest.
Conversely, during cardiac arrest it is unlikely that a drug running by infusion pre-arrest would assist the conduct of the cardiac arrest by continued administration. Sedatives and anaesthetic medications such as propofol are vasodilators and their cessation for a few minutes in the context of very low cerebral perfusion is unlikely to cause awareness. In addition, once stability has been achieved and adequate cerebral perfusion restored, recommencing this infusion will be straightforward. Thus during an arrest after cardiac surgery, stopping all the syringe drivers and infusions may be regarded as best practice.
3.7 Administration of adrenaline (epinephrine)
Evidence was sought for whether routine adrenaline administration may be either useful or potentially harmful for patients who arrest after cardiac surgery. This search is fully documented in the ICVTS [31], together with a summary of all identified papers. We found 889 papers and all major international guidelines were included. Of these, 17 presented the best evidence to answer the clinical question.
Many of the cohort studies reporting the outcome of arrests after cardiac surgery mentioned that adrenaline was given to some patients, but reporting was not consistent or complete enough to conclude that this was either a useful or harmful intervention.
The 2005 European Resuscitation Council guidelines [4] and the American Heart Association guidelines [32] state that for patients suffering a cardiac arrest with pulseless electrical activity (PEA) or asystole, 1 mg of adrenaline should be given as soon as intravascular access is achieved and for every 3–5 min or every other loop of the algorithm. For VF/VT, adrenaline should be given after the second failed shock. However, the ERC states that ‘despite the widespread use of adrenaline during resuscitation, and several studies involving vasopressin, there is no placebo-controlled study that shows that the routine use of any vasopressor at any stage during human cardiac arrest increases survival to hospital discharge. Current evidence is insufficient to support or refute the routine use of any particular drug or sequence of drugs. Despite the lack of human data, the use of adrenaline is still recommended, based largely on animal data.’
The evidence for above recommendation is the worksheets produced by Long and Paradis [33] and Wenzel [34]. Long concludes that adrenaline use is supported by recent animal studies but that no human studies compare it to placebo. Adverse consequences are also noted and subsequent doses are less effective. The level of evidence for adrenaline for VF, PEA or asystole is classified as ‘indeterminate’.
Cairns and Niemann [35] studied 14 dogs who had VF for 7.5 min prior to resuscitation attempts. Three dogs survived and adrenaline increased their coronary perfusion pressure (CPP) by 21 ± 11 mmHg. However, in the remainder, adrenaline only increased the CPP by 3 ± 2 mmHg and subsequent doses had minimal effect on CPP. Klouche et al. [36] used various drugs in 20 rats after VF arrest and found that adrenaline impaired post-resuscitation myocardial function more than vasopressin and a selective alpha-agonist, and this function was similar to saline-placebo controls. However, survival was superior with adrenaline than with controls. Lindberg et al. [37], in 18 pigs with VF after sternotomy and closure, showed that while adrenaline (or noradrenaline) increased CPP during the arrest up to 45 mmHg compared to only 7 mmHg for controls, it significantly impaired cardiac output and oxygen delivery after successful resuscitation. Chen et al. [38], in 47 rabbits with arrest after ET tube clamping, found that adrenaline increased CPP from 4 to 38 mmHg whereas vasopressin failed to do this. Half the rabbits given adrenaline survived compared to 10% of the vasopressin group. Ristagno et al. [39] showed significantly worse cerebral blood flows and oxygenation with adrenaline compared to vasopressin in 10 pigs after cardiac arrest. Vandycke and Martens [40] performed a meta-analysis of five RCTs of high-dose versus standard-dose adrenaline. They found better return of spontaneous circulation but no difference in survival to hospital admission and a poorer outcome to hospital discharge with higher doses of adrenaline. Biondi-Zoccai et al. [41] performed a meta-analysis of vasopressin versus adrenaline in cardiac arrest, finding only two human studies but demonstrating superiority of vasopressin across 33 animal studies. Zhong and Dorian [42] performed a review of adrenaline and vasopressin in cardiac arrest stating that adrenaline had many adverse effects after resuscitation including myocardial dysfunction, worsening arrhythmias and increased myocardial oxygen demand and that human studies in this area were urgently needed. Holmberg et al. [43] surveyed 11,000 patients with out-of-hospital arrest and found that adrenaline was a predictor of adverse outcome for asystolic and VF arrests. Behringer et al. [44] reported that in 178 patients who survived an out-of-hospital arrest, adrenaline cumulative dose had been much higher in those patients with a poor neurological outcome. The best human study in this area compared vasopressin with adrenaline but had no placebo group. Wenzel et al. [45] randomised over 1000 patients with out-of-hospital arrest to vasopressin or adrenaline. There was no difference in VF arrests but patients with asystole or patients who had combined vasopressin and adrenaline showed better survival to hospital admission. Pytte et al. [46] was struck by the fact that the benefit of adrenaline seen in experimental studies had not translated into clinical studies and hypothesised that this may be due to the difference between clinical CPR and the CPR obtained in a laboratory by hydraulic-compression devices. They compared these types of CPR and found that while lab CPR produced significant haemodynamic effects with adrenaline, no haemodynamic increases were seen with clinical CPR. Peak adrenaline level took 2.5 min to achieve in the clinical CPR group after a single administration.
In the cardiac surgical literature Webb [47] reported the case of a patient who lost cardiac output due to a tension pneumothorax. Adrenaline (100 mcg) was administered after needle thoracocentesis failed to return spontaneous circulation. A chest drain restored cardiac output but extreme hypertension then caused massive blood loss and a PEA arrest. Emergency resternotomy and control of the bleeding aortotomy site led to patient survival and eventual successful hospital discharge. This case demonstrated the dangers of adrenaline administration when reversible causes exist in patients after cardiac surgery.
In summary, the European Resuscitation Council and the American Heart Association both recommend 1 mg of adrenaline immediately in PEA/asystole or after the second failed shock in VF/VT. However, they acknowledge the weakness of the evidence behind this recommendation, based entirely on animal studies, which have not been successfully replicated in humans, and grade such evidence as ‘indeterminate’. When arrest follows cardiac surgery, the chances of rapidly restoring cardiac output are good, and routine use of adrenaline in the arrest may result in dangerous subsequent hypertension.
We therefore cannot recommend adrenaline to be given routinely in all such cardiac arrests. We recommend that administration of adrenaline to be delayed until reversible causes of arrest are excluded. We acknowledge that adrenaline may be useful in the impending arrest or peri-arrest situation and may also be safely used in smaller doses such as 100–300 mcg boluses. However, once cardiac arrest has happened, we recommend that adrenaline is only administered by senior clinicians with experience of its routine use and it should not form part of the arrest protocol.
3.8 Cardiac arrest in patients with an intra-aortic balloon pump
Patients with an intra-aortic balloon pump present special considerations. VF or asystolic arrest is normally easy to recognise, but in PEA or in asystole with an active pacemaker, the ECG may continue to trigger the IABP and the arterial waveform may remain pulsatile even in the absence of cardiac output. Cardiac arrest can be confirmed by the loss of the cardiac component of the IABP pressure trace and, more visibly, by the loss of pulsatility in other pressure waveforms such as CVP, PA and pulse oximetry, in which case cardiac arrest should be called.
In an arrest, ECG recordings are either absent or highly variable and subject to artefact from chest compression and other activity. ECG trigger for the IABP is therefore not helpful. Pressure trigger, however, will coordinate diastolic balloon inflation with cardiac compression and may help improve mean blood pressure including coronary artery perfusion pressure. Once cardiac arrest is established, the IABP should therefore be set to pressure trigger, with 1:1 counterpulsation at maximal augmentation. This will allow augmentation of cardiac massage and improved cardiopulmonary resuscitation, without interference from the ECG trace. If there is a period with no cardiac output and no cardiac massage, the IABP may be set to internal trigger at 100/min.
3.9 Management of the cardiac arrest
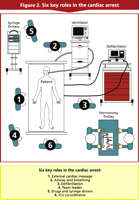
We have identified six key roles in the cardiac arrest situation after cardiac surgery and evaluated them in manikin simulation [20]. Training should be given in these six key roles. When the arrest occurs, each role should be assumed by appropriately trained individuals (Fig. 2 ).
External cardiac massage
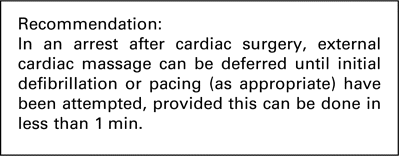
Once the arrest has been established one person is allocated to ECM. This should commence immediately at a rate of 100 beats/min while looking at the arterial trace to assess effectiveness. The only exception to this is when immediate defibrillation or pacing is appropriate prior to ECM.
Airway and breathing
The oxygen must be turned up to 100% and airway and breathing checked as per protocol, specifically to exclude pneumothorax, haemothorax or endotracheal tube problem.
Defibrillation
The defibrillator should be connected and shocks administered if required. This person should also check the pacing, and if emergency resternotomy is being performed, should ensure that internal defibrillators are available and connected.
Team leader
This senior person should conduct the arrest management, ensuring that the protocol is being followed and that there is a person allocated to each role.
Drug administration
This person stops all infusions and syringe drivers and administers atropine, amiodarone and other drugs as appropriate.
ICU co-ordinator
This role, by a senior member of ICU staff coordinates activity peripheral to the bedside. This includes preparing for potential resternotomy as soon as an arrest is called, managing the additional available personnel and calling for expert assistance if not immediately available while continually reporting progress to the team leader.
4 Ventricular fibrillation or pulseless ventricular tachycardia
4.1 The use of a precordial thump in cardiac arrest
We have found no documented papers reporting the success of a precordial thump in patients after cardiac surgery. However anecdotally we have heard reports of success with this intervention. The ERC guidelines of 2005 [3] on the precordial thump [48] report case series with successful cardioversion, particularly of pulseless VT if a precordial thump is administered within 10 s of the onset of the arrhythmia. Thus we recommend a precordial thump if VF or pulseless VT is witnessed, within 10 s of the arrhythmia.
4.2 Immediate external cardiac massage versus immediate defibrillation or pacing
Evidence was sought for whether an initial period of ECM before defibrillation or pacing might benefit the patient or cause unnecessary harm. A search for the evidence on immediate versus delayed ECM is fully documented in the ICVTS [49], together with a summary of all identified papers. Altogether 550 papers were found in Medline and 990 in Embase using the reported search, of which 22 represented the best evidence to answer the clinical question.
4.2.1 Studies on timing of defibrillation
In 2005, the ILCOR task force [24] recommended that for out-of-hospital arrests, where the response time is more than 4–5 min, a 1.5–3 min period of ECM may be of benefit. The task force also stated that there is no evidence to support or refute the use of CPR before defibrillation for in-hospital cardiac arrest. This is based on work by Gazmuri et al. [50] who, as part of the ILCOR review process, performed a systematic review of 14 papers including 2 randomised trials, 2 cohort studies, and 10 experimental studies. The experimental studies showed that defibrillation first is more effective than ECM first if the duration of untreated ventricular fibrillation is 5 min or less.
Of the randomised studies, Wik et al. [51] reported on 200 patients who suffered an out-of-hospital VF arrest randomised to defibrillation then ECM or 3 min of ECM then defibrillation. There was no difference between groups if the response time was less than 5 min. However in the remaining patients, the return of spontaneous circulation was 58% in the ‘ECM first’ group compared to 38% in the ‘defibrillation first’ group.
In the second RCT by Jacobs et al. [52], 256 patients who suffered a VF arrest were randomised to defibrillation first or defibrillation after ECM for 90s. The mean response time was 9 min but no differences in either group were shown in resuscitation or survival.
Of the cohort studies, Cobb et al. [53] in Seattle looked at the implementation of automated external defibrillators (AEDs) for non-paramedic emergency medical teams in over 1000 arrests. In the first period of the study there was no improvement in survival, which led to a change in practice with the recommendation of 90s of CPR before defibrillation. This led to an increase in survival to discharge from 24% prior to the change to 30% with CPR before defibrillation. Of note in the group where response time was over 4 min, this improvement was from 17% prior to the change to 27% with CPR first.
Stotz et al. [54] reviewed the implementation of AEDs in Basel and found that survival to discharge dropped from 24% to 14% after implementation of early defibrillation instead of CPR.
The largest study of in-hospital arrests by the AHA national registry of cardiopulmonary resuscitation [55] was in 2008 with reported data on 6789 patients who suffered an in-hospital VF arrest across 369 hospitals. Most (61%) of arrests were in ICU and 10% were in patients after cardiac surgery. The best survival was in patients defibrillated in under 2 min (39%). There was a significant reduction in survival if defibrillation was beyond 2 min (22%) and prognosis worsened the longer defibrillation was delayed.
Spearpoint et al. [56] reported that in 2 years of VF arrests in 124 patients at the Hammersmith hospital, survival to discharge was 48% with defibrillation within 2 min compared to 14% if defibrillation was delayed. Of 15 patients who had defibrillation without CPR, 12 (80%) survived to discharge.
Fredriksson et al. [57] reported 910 consecutive arrests with 34% survival to hospital discharge. This was thought to be partly due to rapid defibrillation with a median of 2 min. Hajbaghery et al. [58] reported 206 patients who had cardiac arrest in an Iranian hospital. Survival to discharge was 33% if defibrillation was within 4 min, but only 5% otherwise. Zafari et al. [59] reported 569 in-hospital arrests with only 2.2% survival in defibrillated patients. After initiation of an early defibrillation programme this increased to 16%. Skrifvars et al. [60] analysed risk factors for survival at 12 months among 441 patients with in-hospital arrest. Although arrests in a cardiac surgical unit had a better outcome, delay to defibrillation was not a predictor. Apart from the 15 patients described by Spearpoint et al. [56], none of these in-hospital studies included patients who had CPR deferred until after defibrillation.
4.2.2 Reports on trauma induced by ECM
Bohrer et al. [28] reported three patients who suffered a VF arrest after cardiac surgery. They all had brief periods of external CPR and died from massive haemorrhage resulting from mechanically induced disruption of vascular sutures lines. One patient had only five compressions before 1500 ml of blood suddenly came down the drains in 30 s.
Kempen and Allgood [61] reported a right ventricular tear secondary to external CPR in a patient who had cardiac arrest shortly after a right pneumonectomy. We also identified five case reports of cardiac damage due to ECM in the non-surgical literature [62–66]
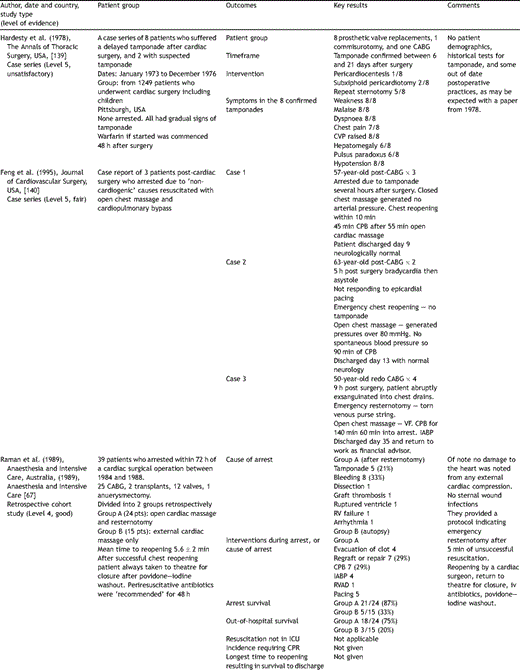
Several cohort studies report the results of cardiac arrest after cardiac surgery but none mention significant complications due to the external CPR. El-Banayosy et al. [14] reported 113 patients who had 20 or more minutes of ECM, with a survival of 70% without any ECM-attributable complications. Raman et al. [67] reported 39 patients with cardiac arrest after cardiac surgery. Emergency resternotomy was performed in 24 and the authors specifically stated that ‘no significant damage to the myocardium was considered to have occurred as a result of direct cardiac compression.’
We found no studies reporting cohorts of patients resuscitated primarily by external pacing or temporary wire pacing. As this intervention is no more invasive than defibrillation, guidance on its timing in relation to ECM in asystole will parallel the timing recommendation for defibrillation in VF. In both cases, delay in obtaining the equipment is an indication for immediate ECM.
In summary, most evidence supporting immediate CPR before defibrillation or pacing derives from out-of-hospital arrests. Survival after in-hospital arrest is favoured by immediate defibrillation. After cardiac surgery, ECM is associated with potentially fatal complications, and may not be necessary in situations where the arrest can be immediately reversed by defibrillation or pacing. We therefore recommend that if defibrillation or pacing (as appropriate) can be performed within 1 min then it is acceptable to defer ECM until they have been attempted.
4.3 Number of attempts at defibrillation before resternotomy
Evidence was sought for the optimal number of attempts at external defibrillation for VF before proceeding to emergency resternotomy. This search is fully documented in the ICVTS [68], together with a summary of all identified papers. Altogether 1183 papers were found in Medline of which 15 represented the best evidence to answer the clinical question.
Current ERC guidelines [2] state that 2 min should be left between attempts at cardioversion for patients in VF or VT. However, after cardiac surgery, prompt chest reopening is known to improve outcomes so that waiting for 2 min between each shock may result in a delay that may impair outcome should defibrillation prove unsuccessful.
In eight studies [69–76], monophasic shocks were compared to biphasic shocks, and in all these papers, biphasic shocks were found to be more successful or equivalent to monophasic shocks. In five of these comparative studies [69–71,75,76], success at the first attempt at defibrillation was between 86% and 98%. In contrast, two of the studies [72,73] showed relatively lower first shock success rates ranging from 17% to 23%. However, in one of the latter studies by Schwarz et al. [73], intra-operative internal shocks were delivered during cardiac surgery on 91 patients, therefore, the first shock energy was lower at 2 J compared to the higher energies (100–150 J) used for the transthoracic delivery in the other studies.
Two animal studies were performed. Cammarata et al. [77] induced VF in 60 pigs, then delivered three sequential 150 J biphasic shocks. The first shock success was 80%, which steeply declined to 15% for the second shock and further dropped to 5% for the third. The second study was by Nieman et al. [78] who induced VF in 38 pigs, who either received three escalating monophasic shocks (200–300–360 J) or fixed biphasic shocks (150 J). Both shock waveforms displayed a similar reduction in shock success from first to third shocks. The first shock success was 50% for biphasic shocks, followed by 30% for the second shock and 5% for the third. Both papers therefore can be taken to imply that the fourth shock success would be below 5%. Accepting the limitation of a pig model, it would seem that routinely proceeding to a fourth shock is unlikely to be beneficial. These results suggest that up to three shocks should be delivered to patients in VF/VT, but after three shocks the chance of successful defibrillation is small.
The sources of data on the number of shocks are wide-ranging and include papers on ICDs, electrophysiological studies, out-of-hospital arrests and animal studies. Furthermore, the rate of success of a second shock after 2 min of CPR has not been reported in any paper that we found. When data from all 15 papers are combined, the average success rate of sequential shocks declines from 78% for the first shock to 35% for the second shock and 14% for the third. Data on fourth shock success was only recorded in one paper [73]. Thus the likelihood of successful cardioversion declines dramatically from first to second shock, and declines further from second to third shock. Mackay et al. [12] reported the results of 79 chest reopenings over 6 years and found that the major determinant of survival was chest reopening within 10 min. We conclude that proceeding to resternotomy after the third shock is preferable due to the minimal chance of fourth shock success.
4.4 Amiodarone
Evidence was sought as to whether amiodarone or lidocaine may be useful if the patient is in VF and not responding to cardioversion. This search is fully documented in the ICVTS [79], together with a summary of all identified papers. Altogether 434 papers were found of which 8 represented the best evidence to answer the clinical question.
Only two articles were found on cardiothoracic patients. A non-randomised retrospective report reported that lidocaine did not prevent ventricular tachyarrhythmias in patients after cardiac surgery [80] and the second article describes a case report in a neonate after corrective cardiac surgery in which the use of amiodarone, in addition to cardioversion, resulted in successful treatment of recurrent ventricular fibrillation [81]. Further evidence supporting the use of amiodarone in patients after cardiac surgery is not available.
According to both European and American 2005 resuscitation guidelines [4,82], no evidence is available that any antiarrhythmic drug given routinely during human cardiac arrest increases survival to hospital discharge. In contrast, two large randomised trials, the ARREST [83] and ALIVE [84] trials, have shown that, in patients with out-of-hospital cardiac arrest, survival to hospital admission is improved by the use of amiodarone as compared to placebo or lidocaine. In the ARREST trial [83], patients with out-of hospital cardiac arrest, amiodarone had a significant increase of 10% in survival to admission (44% vs 34%) with amiodarone when compared to placebo. In the ALIVE trial [84], survival to hospital admission was significantly increased if patients were treated with amiodarone compared with lidocaine (22.8% vs 12.0%, p = 0.009). In addition, more patients developed asystole after defibrillation in the lidocaine group (29% vs 18%, p = 0.04).
Other studies have also shown a consistent improvement in defibrillation response when amiodarone was given to humans or animals with VF or haemodynamically unstable VT [85–89]. Somberg et al. showed in a double-blinded study that amiodarone is more effective than lidocaine in the treatment of shock-resistant VT (immediate VT termination in 78% vs 27%, respectively, p ≪ 0.05) and that hypotension was less frequent than with lidocaine [88]. Anastasiou-Nana induced VF in dogs by coronary artery ligation [90] and found that amiodarone significantly improved defibrillation outcomes when compared to lidocaine. Finally, according to two worksheets of the ILCOR, amiodarone can be safely and effectively used in both paediatric and adult patients with refractory ventricular fibrillation [91,92]
The findings of all above mentioned studies have led to ERC consensus that ‘amiodarone should be considered as the first line antiarrhythmic drug that should be given to patients with VF/pulseless VT that persists after 2–3 shocks plus adequate CPR and use of a vasopressor’ [4,82]. It should be given as a bolus injection of 300 mg. A further dose of 150 mg may be given for recurrent or refractory VF/VT followed by an infusion of 900 mg over 24 h. Lidocaine 1 mg/kg may be used as an alternative but only if amiodarone is not available.
4.5 Automated external defibrillators (AEDs)
For non-cardiac surgical patients, AEDs have been recommended to facilitate defibrillation in hospitals [93] although the studies in animals and manikins were variable and often showed a delay in defibrillation. Only one case report of AED use in cardiac surgery was found [94] but this was for cardioversion of a patient on cardiopulmonary bypass (CPB). In cardiac surgical patients the importance of rapid cardioversion or immediate resternotomy has already been emphasised. AEDs will not deliver three shocks as rapidly as trained clinicians and thus we do not recommend AEDs for use in cardiac surgical patients in the ICU as this may delay the decision to perform resternotomy.
4.6 Pads versus paddles
No studies were found comparing pads to paddles in patients after cardiac surgery. The ERC recommends that either is acceptable [95]. We also recommend that either is acceptable, but that only one or the other be selected in a particular ICU and that staff are trained in its use. Interchanging between paddles and pads is likely to introduce delays in cardioversion during a cardiac arrest and the availability of both in one unit is unnecessary.
4.7 Automatic external compression devices
These devices are available in some hospitals but have not yet been tested on patients after a sternotomy. They should not be used in cardiac surgical patients until their safety in this context can be demonstrated.
5 Cardiac arrest with ‘non-shockable’ rhythm
5.1 Pacing
The initial arrest rhythm will be only amenable to defibrillation in 30–50% of patients. The remainder have other rhythms, which cannot be treated by defibrillation. Of these, predominant rhythms that may be amenable to pacing are severe bradycardia or asystole (Fig. 1).
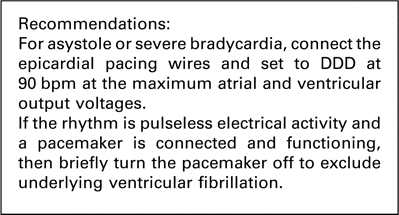
If epicardial pacing wires are in place, they should be immediately connected to a pacemaker. This pacemaker should be set to DDD at a rate of 90 beats/min. The atrial and ventricular output should be set to maximum. If this fails to restore cardiac output, or if there is delay in obtaining pacing equipment beyond 1 min, basic life support must be commenced immediately.
Many pacing boxes have emergency settings using a single button press. It is acceptable to use your emergency setting if you are well trained in its use. However this setting often changes the pacemaker to V00. Clinicians should be aware that dual chamber pacemakers are frequently connected to atrial wires only after cardiac surgery where ventricular wires are not used. Thus V00 would not send an impulse down the atrial wires and clinicians could potentially be misled into thinking that the wires are not functioning if the emergency setting is used. We therefore recommend dual chamber emergency pacing in cardiac arrest. Furthermore we recommend DDD rather than DOO as the pacing box will often already be in this mode and require fewer changes by the operator. In addition we do not believe that the sensing mode will inhibit impulses when the box is turned on to treat extreme bradycardia as part of the resuscitation protocol.
In the absence of epicardial pacing wires, pacing can be achieved using external pacing pads. No studies have reported successful use after cardiac surgery (Table 1 ) but this does not exclude it as a possible intervention if suspicion that the arrest is due to nothing more than extreme bradyarrhythmia. We have included this intervention lower down in the protocol, after ECM has been commenced, due to the additional complexity in setting up external pacing and clinicians’ unfamiliarity with this intervention that we have observed in our manikin simulations even after training.
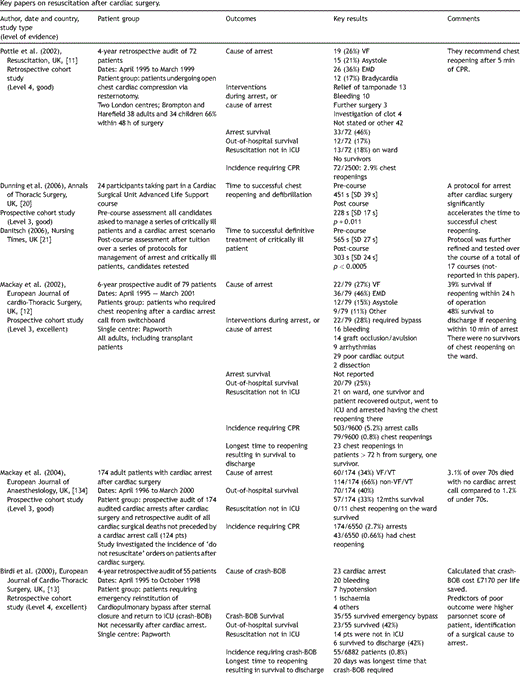
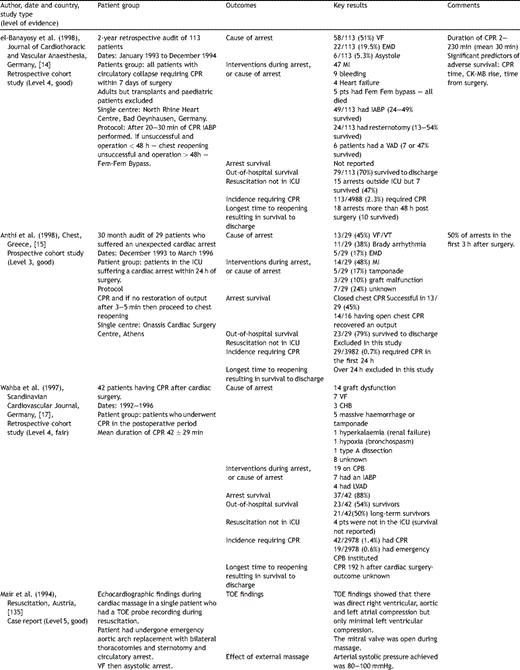
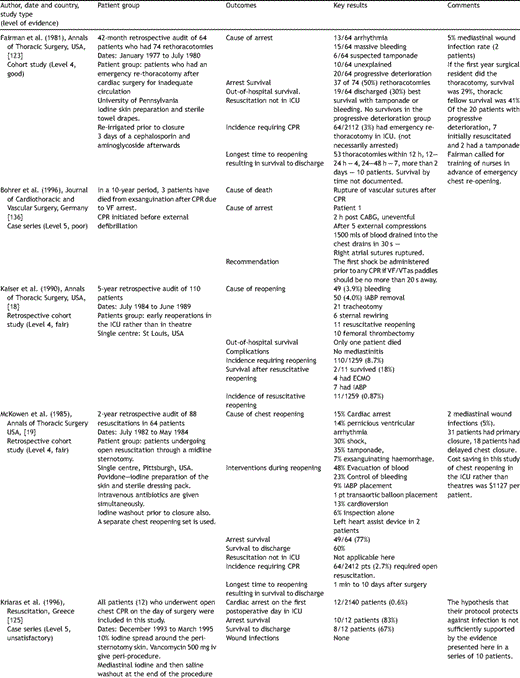
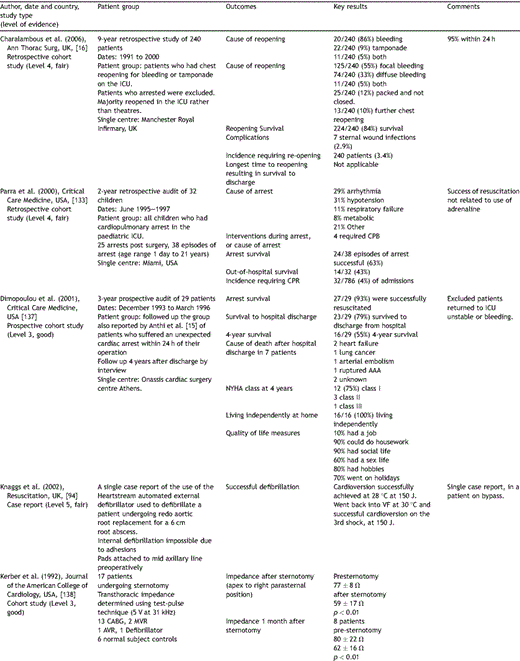
Key papers on resuscitation after cardiac surgery.
If the pacemaker is connected and functioning before the arrest, and the patient has arrested with an ECG showing PEA at a rate that looks like a paced rhythm, the pacemaker spikes on the monitor may disguise underlying VF. We recommend that in this situation it is worth turning off the pacemaker to exclude underlying VF.
5.2 Atropine
Despite widespread use of atropine in all cardiac arrest protocols, its benefit is not well established [96,97]. Five prospective non-randomised controlled trials in non-cardiac surgical patients have failed to establish a survival benefit for in-hospital or out-of-hospital cardiac arrest.
We were unable to find any further evidence in favour of atropine in the cardiac surgical literature. However, atropine is a relatively benign drug with few side-effects and thus in the context of asystole or extreme bradycardia without a viable arterial blood pressure we continue to recommend 3 mg of atropine administered as a single dose via the central line as soon as a non-VF/VT arrest has been identified.
5.3 Emergency resternotomy after non-VF/VT arrest
In non-cardiac surgical patients, non-VF/VT arrests are associated with a poorer outcome. The ERC guidelines ask clinicians to consider the following as causes of the arrest: hypoxia, hypovolaemia, hypo/hyperkalaemia, hypothermia, tension pneumothorax, thrombo-embolism, tamponade and toxins, the so-called four ‘H’s and four ‘T’s.
In contrast, cardiac surgical patients who have a non-VF/VT arrest with this rhythm may have tamponade, tension pneumothorax or severe hypovolaemia. Prompt treatment is associated with an excellent outcome. Thus delays to resternotomy should be minimised.
After connecting the pacemaker and administrating atropine, there should be no delay to the decision to perform emergency resternotomy. If each of the four ‘H’s and four ‘T’s are considered in turn it is apparent that none of these should delay resternotomy. Reversible causes of hypoxia should already have been addressed as part of the basic life support protocol already described. Hypovolaemia, which causes arrest, will inevitably require a resternotomy to stem the bleeding. Hypo/hyperkalaemia are unlikely causes of arrest after cardiac surgery as serum potassium is regularly monitored but if this is the cause of the arrest, a prolonged period of CPR may be needed which will be better performed by internal massage. Hypothermia causing arrest is unlikely and may require active rewarming on bypass as passive rewarming would already have been used in the ICU prior to the arrest. Tension pneumothorax should be identified by the assessment of the airway and breathing during basic life support and treated by drainage. If undetected clinically, it will be promptly relieved by emergency resternotomy. Tamponade requires resternotomy and is the commonest cause of non-VF/VT arrest after cardiac surgery. Toxins are unlikely and cessation of infusions may be all that can be done unless clinical suspicion regarding a specific drug is raised. Finally thromboembolic and mechanical obstruction such as a pulmonary embolus or obstructed valve will be difficult to treat other than with emergency resternotomy.
Thus prompt resternotomy should be performed if there is a non-VF/VT cardiac arrest, which does not resolve with atropine and epicardial pacing.
6 Conduct of emergency resternotomy
6.1 Internal versus external cardiac massage
Evidence was sought to compare the efficacy of internal to external cardiac massage. This search is fully documented in the ICVTS [98], together with a summary of all identified papers. Altogether 527 papers were found in Medline of which 15 represented the best evidence to answer the clinical question.
ILCOR [99] provided a systematic review of the topic as part of the worksheet review process [100]. They found 4 human studies, with 2 in cardiac surgery and 2 in out-of-hospital cardiac arrest and 18 animal studies. They report benefits of internal cardiac massage including better coronary perfusion pressure, increased return of spontaneous circulation, superior organ blood flow and better survival rates as compared to ECM. They recommend that open chest CPR should be considered for cardiac arrest in the early postoperative phase after cardiothoracic surgery or when the chest or abdomen is already open.
The two human studies after cardiac surgery were by Anthi et al. [15] and Pottle et al. [11]. Anthi reported 29 patients who had cardiac arrest less than 24 h after cardiac surgery, of whom 45% were resuscitated with closed chest CPR and 48% with open chest CPR after 2–5 min of closed chest CPR had failed. Pottle reported 72 patients who had open chest CPR after cardiac surgery, of whom 46% regained spontaneous circulation. Boczar et al. [101] studied 10 patients brought into hospital with a witnessed cardiac arrest. After 5 min of closed chest CPR they were declared unsalvageable and entered into the study. A left lateral thoracotomy was performed without opening the pericardium and internal massage commenced. The mean coronary perfusion pressure rose from 7.3 mmHg to 32 mmHg, the compression phase pressure gradient rose from 6.2 mmHg to 32.6 mmHg and three patients achieved a spontaneous circulation. Paradis et al. [102] in JAMA reported that 15 mmHg of coronary perfusion is required in humans to obtain a return of spontaneous circulation and that this was achieved in all Boczar’s patients during open chest CPR. Paradis et al. [103] also reported three patients who had open chest CPR after failed closed chest CPR and two survived, one with no neurological deficit.
Takino and Okada [104] compared 26 patients who had open chest CPR after witnessed out-of-hospital cardiac arrest with 69 who only had closed chest CPR. Return of spontaneous circulation was 58% of patients had with open chest CPR compared with 30% with closed chest CPR. There were three open chest long-term survivors compared to only one closed chest survivor. A third human study by Hachimi-Idrissi et al. [105] found that in 33 out-of-hospital arrest patients who had open chest CPR after failed closed chest CPR, 13 had spontaneous return of circulation but only 2 survived.
We identified three additional human studies. Del Guercio et al. in 1965 [106] showed significant improvements in physiological variables with open chest CPR in 11 patients with in hospital arrest. The cardiac index was 0.6 l/min m2 with closed chest CPR and 1.3 l/min m2 with open chest CPR. The circulation time decreased from 89 s to 44 s. Calinas-Correia and Phair [107] reported seven patients who underwent open chest CPR but with no survivors. Geehr et al. [108] performed an RCT of open versus closed chest CPR in 49 patients with out-of-hospital arrest and found 3 survivors in each group.
Of the animal studies Benson et al. [109] induced VF in 12 dogs and after 5 min of no intervention randomised the dogs to either open or closed chest massage for 15 min. All open chest massage dogs survived without neurological deficit but only three of seven dogs survived after closed chest massage and two had severe neurological deficit. The coronary perfusion pressure was double in the open chest group. Sanders et al. [110] showed that open chest CPR allowed four out of five dog survivors compared to none in their closed chest group. Kern et al. [111] showed that resuscitation and survival were significantly improved with open CPR in 29 dogs and reiterated these findings in 1991, adding that resuscitation was significantly improved if chest opening was instituted sooner. Raessler and Kem [112] showed in 63 mongrel dogs that open CPR had a coronary perfusion pressure of 64 mmHg compared to 21 mmHg for closed CPR. Rubertsson and Grenvik [113] showed similar significant improvements in cardiac index and coronary perfusion pressure in pigs.
Thus 18 good quality animal studies have consistently demonstrated the superiority of open chest cardiac massage, with the cardiac index and coronary perfusion pressures often more than doubling. There are fewer human studies but they have shown that closed chest massage generates a cardiac index of around 0.6 which rises to 1.3 l/min m2 or more with open chest massage, accompanied by even bigger improvements in coronary perfusion pressure. ILCOR recommends prompt conversion to open chest cardiac massage in patients who have cardiac arrest shortly after cardiac surgery. We support this intervention if simple resuscitative efforts such as defibrillation, pacing or atropine fail, in order to improve significantly the quality of cardiopulmonary resuscitation.
6.2 Abdominal compression to achieve external cardiac massage
Evidence was sought to assess the efficacy of abdominal only CPR compared to ECM. This search is fully documented in the ICVTS [141], together with a summary of all identified papers. Altogether 334 papers were found in Medline of which 10 represented the best evidence to answer the clinical question.
Current guidelines from the European Resuscitation Council do not address the role of only abdominal compression (OAC) CPR. We found one study that looked at only abdominal compression CPR without chest compression. Geddes et al. [114] measured the coronary perfusion index of 11 pigs to determine the efficacy of CPR during VF. They were able to show that OAC-CPR was superior to standard CPR by providing more coronary perfusion. This was a small animal study but there have been other studies that have examined the role of other forms of abdominal CPR.
It was the original findings of Ralston et al. [115] that led to interest in abdominal CPR and this study evaluated the use of interposed abdominal (IAC) CPR. There have been three RCTs [116–118] that showed an improvement in outcome measures of IAC-CPR. Trials involving CPR are often small but a meta-analysis of IAC-CPR [119] limited to human clinical trials comparing IAC-CPR to standard CPR showed a statistically significant benefit in favour of the IAC-CPR in return of spontaneous circulation.
Sustained abdominal compression has also been evaluated recently in an animal study [120] that showed a similar increase in coronary perfusion pressure compared to vasopressor drugs. Babbs [121] used computer modelling to show that high frequency abdominal CPR could produce sufficient systemic perfusion pressures during cardiac arrest. No studies reported visceral organ damage during any of the abdominal compression techniques.
Abdominal only CPR theoretically has the potential to provide systemic perfusion while an emergency resternotomy is being performed, but further evidence is needed before it can be recommended for routine use.
6.3 The emergency resternotomy set
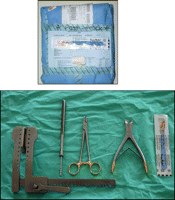
If a resternotomy is to be performed rapidly, ICU staff must be trained in this multi-personnel procedure. One reason for delay in emergency resternotomy is the preparation of a standard sternotomy instrument set [20] which may contain over 30 items of equipment, although only 5 items are essential: an all-in-one sterile thoracic drape, a scalpel, a wire cutter, a heavy needle holder, and a single piece sternal retractor. A Jankauer sucker may be useful and can be connected to the bedside suction point. Larger sets may confuse staff unaccustomed to assisting in theatres and thus cause delay. In addition, when the theatre team arrives, the full thoracic instrument set may have been partially desterilised when opened by the ICU staff in the emergency and have to be replaced.
We recommend every cardiac surgery ICU should be equipped with a small emergency resternotomy set with the disposable scalpel taped to the top (Fig. 3 ). Once the chest has been opened, this set can be discarded and a full set opened in a more measured fashion. This concept is not new and was advocated as long ago as 1985 [19].
Small resternotomy set packed with scalpel on top (above) and opened (below).
6.4 Preparation for emergency resternotomy
Emergency resternotomy is a multi-practitioner procedure, which should ideally be rapidly performed with full aseptic technique. Cardiac arrests in the ICU are often promptly attended by more staff members than needed simply to manage basic life support, defibrillation, airway, and decision making. Therefore additional staff should immediately prepare for resternotomy as soon as cardiac arrest is confirmed, because 20–50% of cardiac arrests after cardiac surgery result in emergency resternotomy [12,17,18]. The minor potential waste in re-sterilising equipment if not needed is more than compensated for by the benefits gained from early resternotomy [20].
Two or three staff members should gown and glove as soon a cardiac arrest is called. Hand washing is time-consuming in an emergency situation and incomplete drying of the hands will slow the donning of gloves. Hand washing is not necessary if an aseptic closed-sleeve technique in donning gown and gloves is used.
6.5 Personnel performing emergency resternotomy
Emergency resternotomy may be required in 0.8–2.7% of all patients undergoing cardiac surgery (Table 1). A cardiac surgeon at registrar (resident) level or above is normally available. However, there may be situations where a surgeon is either unavailable or unable to attend immediately. As resternotomy is often an integral part of successful resuscitation after cardiac surgery, it is beneficial for all personnel who participate in resuscitation in this setting to be aware of the technique of emergency resternotomy and to have practised it. This ensures better assistance for the surgeon and, in the unlikely situation that resternotomy is required and a surgeon is not immediately available, resternotomy by another member staff may be life saving [20–22,122].
6.6 Emergency resternotomy
Don a gown and gloves in a sterile fashion using the closed glove technique. ECM must continue until you are ready to apply the all-in-one sterile thoracic drape.
When ready, ask the person performing ECM to stand aside after removing the sternal dressing.
Apply the thoracic drape ensuring that the whole bed is covered.
If an all-in-one sterile drape is used (as recommended) then there is no need to prepare the skin with antiseptic, as there is a clear adhesive plastic window in the drape to cover the skin. This will not stick if a skin preparation is used (skin preparation only sterilises the skin as it dries which requires several minutes).
Recommence ECM (changeover from non-sterile ECM to sterile ECM should take no more than 10 s).
When equipment is ready (Fig. 3), cease ECM and use the scalpel to cut the sternotomy incision, including all sutures deeply down to the sternal wires.
Cut all the sternal wires with the wire cutters and pull them out with the heavy needle holder. The sternal edges will separate a little and tamponade, if present, may be relieved at this point. This is significantly faster if one person cuts the wires with the wire cutter and a second assistant removes the wires with the heavy needle holder.
Use suction to clear excessive blood or clot.
Place the retractor between the sternal edges and open the sternum. If cardiac output is restored then you have successfully treated the cardiac arrest and should wait for expert assistance.
If there is no cardiac output, carefully identify the position of any grafts and then perform internal cardiac massage and internal defibrillation if required.
6.7 Method of internal cardiac massage
This is potentially dangerous and personnel who may be required to perform this must have had prior training to do this safely. Risks to the patient include avulsion of a bypass graft, with the left internal mammary artery being at particular risk. If you are inexperienced you should not rush to perform internal cardiac massage once the chest is open. It is more important to carefully remove any clot and identify structures at risk such as grafts prior to placing your hands around the heart.
Single hand techniques may disrupt the right ventricle especially if it is thin or distended. There are several methods of internal massage and if you are experienced then you may use the technique that is most suitable for the particular scenario. In our view the safest method for people who do not routinely handle the heart is the two-hand technique.
Before attempting internal massage, inspect the heart to locate the internal mammary and any other grafts, carefully removing any blood clots. Pass the right hand over the apex of the heart (minimising the likelihood of avulsing any grafts, as grafts are rarely placed near the apex). The right hand is then further advanced round the apex to the back of the heart, palm up and hand flat. The left hand is then placed flat onto the anterior surface of the heart and the two hands squeezed together. Flat palms and straight fingers are important to avoid an unequal distribution of pressure onto the heart, thereby minimising the chance of trauma. If there is a mitral valve replacement or repair, care should be taken not to lift the apex by the right hand, as this can cause a posterior ventricular rupture. Squeeze your hands together at a rate of 100 per min and look at the arterial trace to verify adequate internal massage. You should try to obtain a systolic impulse of more than 60 mmHg.
6.8 Cardiac arrest protocol and emergency resternotomy outside the ICU
Emergency resternotomy outside of the ICU is associated with a poor survival [11,12] although occasional patients do survive [13,14]. Postoperative wards may not only care for patients after cardiac surgery, but may also have thoracic surgical or medical patients.
It is important that members of a resuscitation team use the same resuscitation protocol. The guideline presented here is not appropriate for patients who have not recently undergone cardiac surgery via sternotomy. Together with the fact that emergency resternotomy is less effective outside ICU, we recommend that this guideline is not used on the ward and the ERC guidelines should be used [4]. However arrangements should be made locally for experienced cardiac surgical personnel to be immediately available to attend an arrest on the ward.
Local guidelines as to the appropriate location of an emergency resternotomy together with the appropriate personnel to assist should be drawn up to address the situation in which the attending surgeon feels that an emergency resternotomy is required for a patient on the ward. One option is to use the trained personnel from the ICU together with their emergency resternotomy equipment on the ward or alternatively to transport the patient immediately to the ICU or theatre.
6.9 How long after cardiac surgery is emergency resternotomy no longer indicated?
As the patient recovers from cardiac surgery, the chance of a cardiac arrest occurring due to a cause that can be corrected by emergency resternotomy is reduced. Tamponade, graft occlusion and life-threatening arrhythmias mostly occur in the hours after cardiac surgery [11–14,17,123]. However delayed tamponade may still occur later, possibly in association with pacing wire removal or overanticoagulation [124]. In addition, when cardiac arrest occurs several days after cardiac surgery, internal cardiac massage remains a superior method of resuscitation compared to ECM (Section 6.1). Thus, even if a reversible cause such as tamponade is not suspected, emergency resternotomy is preferable to prolonged ECM. However, this must be balanced with the danger of resternotomy once adhesions have started to form. It is the opinion of the committee that adhesions would be unlikely to be present until at least 10 days postoperatively. Therefore emergency resternotomy should form a standard part of the arrest protocol up to the 10th postoperative day. Thereafter emergency resternotomy should be considered but a senior clinician should make the decision as to whether the resternotomy is performed, balancing the risks of damage to increasingly adherent mediastinal structures with the likely chances of a successful outcome to the arrest with emergency resternotomy.
6.10 Sterility during emergency resternotomy
It has been shown that the time to successful emergency resternotomy with a full aseptic technique can be halved if good training is given [122], thus there is no need to compromise complete aseptic technique in order to expedite emergency resternotomy. Furthermore, good aseptic technique has been shown to give acceptably low rates of mediastinitis after emergency resternotomy [16,123,125,126]. The surgeon and all assistants should wear sterile gown and gloves. Facemasks or surgical caps are not essential. We recommend that the optimal technique to maintain complete field sterility is to use a disposable all-in-one thoracic drape with a clear window that covers the skin, which obviates the need for skin preparation. Compromise approaches, such as using sterile gloves only, or non-sterile gloves with the intention of performing a washout after successful resternotomy are not acceptable as simple sterile techniques can be employed without undue delay.
6.11 Cardiopulmonary bypass after emergency resternotomy
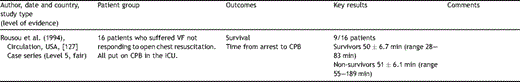
If a spontaneous cardiac output has not been established after emergency resternotomy and internal cardiac massage, a further option is the institution of CPB. We found no papers to guide the technical aspects of the safe passage onto bypass in this special situation although Rousou et al. [127] documented a 56% survival in 16 patients with refractory VF despite open chest CPR who were then placed on CPB in the ICU. After consultation with the Society for Clinical Perfusionists of Great Britain and Ireland we make some best practice recommendations.
There is a concern that the heparin may not circulate fully in an arrest. As soon as the decision is made to use CPB, 30,000 iu of heparin should be immediately administered. In addition, we recommend that 10,000 iu of heparin be added to the bypass machine reservoir. It is not necessary to check an ACT before commencing CPB in the cardiac arrest situation.
Cannulae may be inserted into the aorta and right atrium without purse strings and held by assistants until pursestrings are applied on bypass. Surgeons should be aware that the right atrial pressure will be substantially higher than in routine cannulation and prior connection of the venous cannula to the venous return line will reduce blood loss from this manoeuvre.
6.12 Should patients receive additional antibiotics after emergency resternotomy?
Evidence was sought for whether additional antibiotics reduce the incidence of mediastinitis after emergency resternotomy. This search is fully documented in the ICVTS [126], together with a summary of all identified papers. We found 527 papers, of these, 9 presented the best evidence to answer the clinical question.
In 2007, the Society of Thoracic Surgeons’ guideline on antibiotic prophylaxis for elective cardiac surgery [128] recommended a first-generation cephalosporin (usually cefazolin) as the antibiotic of choice for elective cardiac surgery with the addition of vancomycin for patients at increased risk of staphylococcal infection. The SIGN guideline [129] also recommends antibiotic prophylaxis for patients undergoing cardiac surgery. Neither guideline addresses emergency resternotomy in patients who have recently received these prophylactic antibiotics and may not necessarily have had a sterile reopening. Kriaras et al. [125] published the only paper to look into the issue of antimicrobial protection in patients who had open chest resuscitation after cardiac surgery. Twelve patients had 10% iodine spread around the peri-sternotomy skin and vancomycin 500 mg intravenously. Mediastinal iodine and then saline washout were given at the end of the procedure. There were no wound infections, and they concluded that this protocol might be useful in emergency situations.
McKowen et al. [19] reported the outcomes from resuscitation of 64 cardiac surgical patients after emergency resternotomy. Povidone–iodine skin preparation, intravenous antibiotics and bacitracin washout prior to closure were used and only 2 out of 49 patients had a wound infection (4%).
Charalambous et al. [16] studied patients who had chest reopening for bleeding or tamponade (but not cardiac arrest) on the ICU. The skin was prepared with povidone iodine solution and the prophylactic antibiotic was usually flucloxacillin or similar, with variable duration of administration. The incidence of sternal wound infection was 3%. Anthi et al. [15] reported that betadine was applied to the skin and full sterile technique used in 16 emergency chest reopenings after a cardiac arrest. No patients had a wound infection. Fairman and Edmunds [123] described 64 patients who had an emergency resternotomy after cardiac surgery with iodine skin preparation and sterile towel drapes. Wound irrigation was performed prior to closure, followed by 3 days of a cephalosporin and an aminoglycoside. Wound infection rate was 5% in survivors. El-Banayosy et al. reported the outcomes of 113 patients who arrested within 7 days of cardiac surgery [14] and found that 7 of the 79 survivors had at least one episode of sepsis after the resuscitation (9%). No mention of antibiotic use was given. Ramen et al. [67] reported 39 patients who arrested after cardiac surgery. An emergency resternotomy was performed in 21 patients with povidone–iodine skin preparation, aseptic reopening on the ICU and perioperative antibiotics for 48 h. In addition, patients were returned to theatre for povidone–iodine washout and closure. Several papers documented ‘full aseptic technique’ but no more detail was given. For patients who require an emergency resternotomy on the ICU, the incidence of sternal wound infection or sepsis after this emergency treatment is around 5% in seven papers. Of these, five reported routine additional intravenous antibiotics and an iodine washout. We conclude that the incidence of subsequent infection is low in emergency resternotomy after cardiac arrest, and that full aseptic technique, including gown and gloves, is both indicated and feasible. It is common practice also to give additional antibiotics and an antiseptic washout although we could identify no other comparative studies in support of this.
6.13 Permissive hypothermia after resuscitation from prolonged cardiac arrest
It is well established that patients who have a significant period of cardiac arrest out of hospital should be systemically cooled to 32–34 °C for 12–24 h [130–132]. We found no additional studies in patients after cardiac surgery but as this is well established outside our specialty, this intervention should be considered if it is felt that there has been a significant period of poor cerebral perfusion.
7 Special considerations
There are many special considerations within cardiac surgery related to the specific operative procedures. The cases below serve as examples and every clinician should consider whether the patient that they are returning to the ICU may present a particular challenge should cardiac arrest occur, and if so, this should be clearly documented and discussed with the ICU staff.
7.1 Transplant patients
Patients undergoing heart, heart–lung or double lung transplant via a sternotomy may be resuscitated using these guidelines. Patients having a transplant procedure via a clamshell incision or bilateral thoracotomy incisions should have an emergency re-thoracotomy through the previous particular incision using the same indications in this guideline. Only a surgeon experienced in this particular approach should perform this procedure. Atropine will be ineffective for patients after operations involving a cardiac transplant and therefore this may be omitted from the non-VF/VT protocol.
7.2 Paediatric patients
The only reported series that we identified sets the incidence of cardiac arrest at 4% after cardiac surgery in children [133]. The success of resuscitation is similar to adult patients and the causes are also similar although 11% suffered a respiratory arrest in this series. This guideline should be read together with the ERC guidelines on paediatric cardiac arrest [6]. Paediatric cardiac surgery ICUs may use this protocol but it must be noted that none of the drug dosages are intended for use in children and all dosages must be corrected for body weight or surface area, as is the usual practice for drug administration in paediatrics.
7.3 ‘Open chest’ patients
Occasionally a patient after a high-risk operation will be returned to the ICU with the sternum ‘open’. The heart may be surrounded by gauze packs, especially if bleeding has been difficult to control. Such patients are at high risk for cardiac arrest. The surgeon should hand over specific guidelines for their care should a cardiac arrest occur. However we recommend that they be cared for using this guideline. ECM should be performed at the midpoint of the chest, over the packs, and the arterial pressure trace should be observed to assess the effectiveness of external massage. If emergency resternotomy is then indicated, full aseptic technique should be used, and this will be easier as sternal wires will not need to be removed. In particular the packs may contribute to cardiac compression, inducing an element of tamponade and thus should be carefully removed, making sure that no grafts are adherent to them if present.
7.4 Patients with a cardiac assist device
All clinicians caring for these patients should have full training in the procedures for equipment failure and the ‘cardiac arrest’ situation. The guidelines presented in this document do not apply to these patients. They are highly complicated due to the fact that an ‘arrest’ may be due to mechanical failure and in this situation ECM is not appropriate. A protocol for resuscitation and back-up support must be established and rehearsed.
7.5 Patients undergoing non-sternotomy cardiac surgery
Some cardiac operations avoid a full sternotomy. This may range from a partial sternotomy, port-access surgery with a mini-thoracotomy, minimally invasive coronary artery bypass (MIDCAB) to TECAB (totally endoscopic coronary artery bypass). It is appropriate to follow this guideline and it is important that the ICU has only one protocol for the initial management of a cardiac arrest.
The operating surgeon should however ensure that the staff members are fully aware of how an emergency reopening should be performed should cardiac arrest occur and emergency reopening be indicated. In these cases it is acceptable for the operating surgeon to indicate that a reopening should not occur unless a senior surgeon familiar with the particular operation is present. This should be discussed with the ICU on admission from theatre.
It should be noted that internal cardiac massage is difficult to perform from a right thoracotomy such as that used in port-access mitral surgery, and therefore it is likely that in the event of a cardiac arrest, these patients should receive a sternotomy by an experienced surgeon rather than rethoracotomy. A sternal saw should therefore be immediately available on the ICU for these patients.
Similarly a patient undergoing coronary artery bypass grafting via a MIDCAB incision should probably undergo a sternotomy rather than extending the incision laterally in an arrest. The sternotomy allows full access to the heart and is most familiar to the attending surgeon and team. It should be noted that the LIMA may not have been fully harvested from the chest wall, and extra care should be taken if internal massage or cardiac manipulation is required.
8 Guideline implementation
The transition phase of modifying resuscitation guidelines in the ICU represents a time of high risk to both patients and staff. In particular, there are clear dangers in changing from a single-shock protocol followed by cardiac massage to a three-sequential shock protocol. The change should be discussed in advance as a unit, and ideally training given in advance of practice change. Training resources are available (www.csu-als.com) together with specialist resternotomy manikins, but equally it is hoped that this guideline is sufficiently comprehensive that it may be taken into the ICU and after local discussion and practice, the guideline may be implemented without any further specialist equipment or assistance.
This is an interim guideline prior to full discussion with the International Liaison Committee on Resuscitation who will be updating all resuscitation guidelines in 2010. Thus it is possible that substantial updates will be available at that time.
Acknowledgements
EACTS are grateful for the help from the following people in developing these guidelines on resuscitation: Zulfiquar Adam, Sharil Ariffin, Tara Bartley, Claire Blood, Sameena Choudrey, Debbie Danitsch, Moloy Das, Arosha Dissanayake, Mike Foley, Katy Gofton, Sarah Hodson, John Jerstice, Pia Khan, Boudewijn Leeuwenburgh, Kevin Mackway-Jones, Jacintha Mass, June McKeown, Jay Nandi, Mike Poullis, Brian Prendergast, David Quayle, Lydia Richardson, Helen Rodriquez, Andrew Roscoe, Rex Stanbridge, Hariharan Subramanian, Andrew Tjon, Myrto Tsagkataki, Darragh Twomey, Akbar Vohra, Stephen Webb, Edwin Woo, Elaine Yap.